Pupil diameter tracks the exploration-exploitation trade-off during analogical reasoning and explains individual differences in fluid intelligence
Abstract
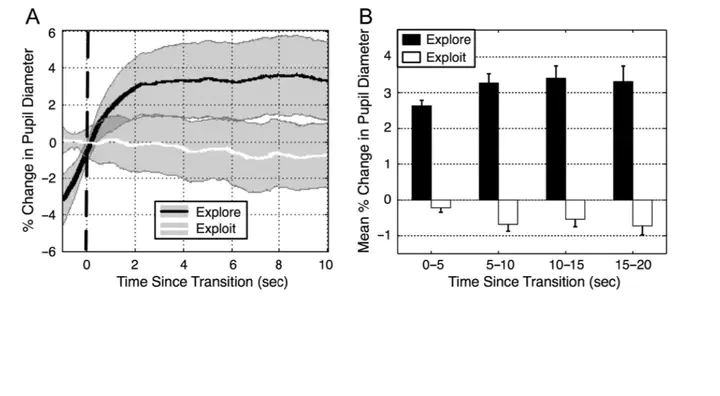
The ability to adaptively shift between exploration and exploitation control states is critical for optimizing behavioral performance. Converging evidence from primate electrophysiology and computational neural modeling has suggested that this ability may be mediated by the broad norepinephrine projections emanating from the locus coeruleus (LC) [Aston-Jones, G., & Cohen, J. D. An integrative theory of locus coeruleus-norepinephrine function: Adaptive gain and optimal performance. Annual Review of Neuroscience, 28, 403–450, 2005]. There is also evidence that pupil diameter covaries systematically with LC activity. Although imperfect and indirect, this link makes pupillometry a useful tool for studying the locus coeruleus norepinephrine system in humans and in high-level tasks. Here, we present a novel paradigm that examines how the pupillary response during exploration and exploitation covaries with individual differences in fluid intelligence during analogical reasoning on Raven’s Advanced Progressive Matrices. Pupillometry was used as a noninvasive proxy for LC activity, and concurrent think-aloud verbal protocols were used to identify exploratory and exploitative solution periods. This novel combination of pupillometry and verbal protocols from 40 participants revealed a decrease in pupil diameter during exploitation and an increase during exploration. The temporal dynamics of the pupillary response was characterized by a steep increase during the transition to exploratory periods, sustained dilation for many seconds afterward, and followed by gradual return to baseline. Moreover, the individual differences in the relative magnitude of pupillary dilation accounted for 16% of the variance in Advanced Progressive Matrices scores. Assuming that pupil diameter is a valid index of LC activity, these results establish promising preliminary connections between the literature on locus coeruleus norepinephrine-mediated cognitive control and the literature on analogical reasoning and fluid intelligence.